
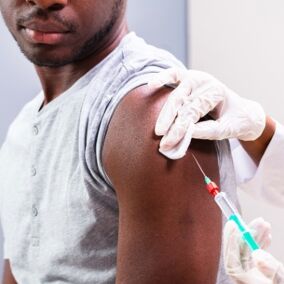
The FDA yesterday approved an injectable form of PrEP. The medication can be administered every two months to help prevent someone from acquiring HIV.
As previously reported by Queerty, ViiV Healthcare, which is majority-owned by GlaxoSmithKline, has developed the injectable PrEP treatment called cabotegravir. It will be marketed under the brand name Apretude.
Anyone wanting to take Apretude must start by having two injections, one month apart. They can then move to an injection every two months.
Before having their first injection, they can take cabotegravir tablets (marketed as Vocabria) for four weeks. This is to check their tolerance of the drug ahead of their first injection.
How about we take this to the next level?
Our newsletter is like a refreshing cocktail (or mocktail) of LGBTQ+ entertainment and pop culture, served up with a side of eye-candy.
Related: FDA says injectable PrEP a ‘breakthrough’ therapy as it pends approval
It’s also vital to test anyone wanting the medication is HIV-negative. The FDA says Apretude will come with a boxed warning about this, as anyone with HIV who takes the drug may develop drug-resistance and limit their future treatment options.

“Today’s approval adds an important tool in the effort to end the HIV epidemic by providing the first option to prevent HIV that does not involve taking a daily pill,” Dr. Debra Birnkrant, the director of antivirals division at the FDA’s Center for Drug Evaluation and Research, said.
“This injection, given every two months, will be critical to addressing the HIV epidemic in the US, including helping high-risk individuals and certain groups where adherence to daily medication has been a major challenge or not a realistic option.”
Deborah Waterhouse, CEO, ViiV Healthcare, said in a press statement: “People who are vulnerable to acquiring HIV, especially those in Black and Latinx communities who are disproportionately impacted in the US, may want options beyond daily oral pills.
“That’s why ViiV Healthcare is proud that Apretude was studied in one of the most diverse and comprehensive HIV prevention trial programs to date, which also included some of the largest numbers of transgender women and Black men who have sex with men ever enrolled in an HIV prevention trial.”

Related: FDA approves first long-acting HIV medication: a monthly injection
The FDA approval comes after a trial on 7,700 participants in 13 countries. That study concluded Apretude was even more effective than daily PrEP pills at preventing HIV infection.
Shipping of Apretude to wholesalers in the US will begin in early 2022.









































Openminded
Any tool to help fight HIV is great news. I still can’t understand how someone has problems adhering to daily pill taking when it is for protection of one’s life. Personally, I’d probably have more trouble maintaining an adherence to getting a shot every 60 days. Maybe we would be closer to an HIV vaccine if the time and dollars spent on R&D for this Prep alternative was spent on HIV vaccine development.
CityguyUSA
Big Pharma wants treatments not cures.
cliche guevara
Seems silly to have to point out the obvious but here we go. PrEP isn’t a treatment, it’s a preventative. It’s literally in the name Pre-ExPosure. It seems that if “Big Pharma” was going for a money grab here they would have the preventative and the cure covered on account of dead people are terrible customers.
BuzzBuzzard
For many, Truvada and its generic make PrEP an unaffordable option. In 2017 I enrolled in a Glaxo program to get Truvada for free, and I take it every day without fail–even though I’m not sexually active on any regular basis. (I was violently raped by six men in Hungary six years ago, so things haven’t been the same since–but undergoing post-exposure treatment in Eastern Europe was a living death.) Because of the US healthcare system, even generic Truvada still costs nearly $1,000 a month. But my insurance covers all of that (and Glaxo did when Truvada was closer to $3,000 a month).
I’m extremely fortunate to have discovered Glaxo’s distribution program and to have insurance. Yet many people are not. Not to mention, many people at the greatest risk of HIV infection–whether because of unhesitant promiscuity, IV drug use, or weak information or education–are loath or unable to take a pill every day. I’m not judging anyone, as I’ve slept around plenty and used to be a junkie.
Getting poked every two months to prevent HIV would be much simpler for me, and I’ll raise the option the next time I see my doctor. (Even on Truvada, I have to get blood work every three months to ensure that I’m negative and not suffering kidney distress.) And, as some of us have been dreaming since HIV emerged in the 1980s (when I was a teen), hopefully a cure will soon be at hand.